Brochure
Tanvex CDMO – GMP Manufacturing Brochure
Read More
›As the global demand for accessible biologics continues to soar, the need for capacity and capabilities in mammalian and microbial derived therapeutics development and production has never been more critical. At Tanvex, we rise to this challenge.
Who We Are
Pairing our proven track record of successfully advancing biologics from Investigational New Drug (IND) application to Biologics License Application (BLA) with our state-of-the-art facilities and high-throughput workflows, our expert team is poised to serve as your collaborative partner. Together, we can deliver groundbreaking advancements in biopharmaceuticals and make a lasting impact on healthcare worldwide.

Facilities
Piloted by a team of approximately 100 biologics experts, our GMP facility in San Diego, California’s Sorrento Valley offers over 100,000 square feet of mammalian and microbial manufacturing capacity and is fully equipped with state-of-the-art single-use technology. As one of the few full-service CDMO campuses on the West Coast, our facility provides convenient CDMO access to California’s thriving biotech hub.
Analytical development and method qualification/validation
Drug substance process development and optimization
Toxicology material production
Mammalian and Microbial GMP manufacturing
Formulation development and drug product testing
Quality control and lot release testing
ICH compliant stability studies
IND to BLA regulatory support
GMP warehousing

Contract Development
Optimize your biopharmaceutical program with Tanvex’s unparalleled contract development capabilities. Our expertise spans the spectrum, from seamless technology transfers to the precision of cell line development. With a focus on both mammalian and microbial process development, Tanvex empowers your vision with cutting-edge analytical insights and transformative formulation and drug product development, laying a strong foundation for your program’s future success.

Contract Manufacturing
Elevate your biopharmaceutical program with Tanvex’s state-of-the-art contract manufacturing suite. Specializing in both mammalian and microbial systems, our team delivers solutions embodying excellence at every step. Our commitment to quality control and assurance ensures that your product meets the highest standards of efficacy and safety, while integrated supply chain management systems work to bring your vision from concept to reality.
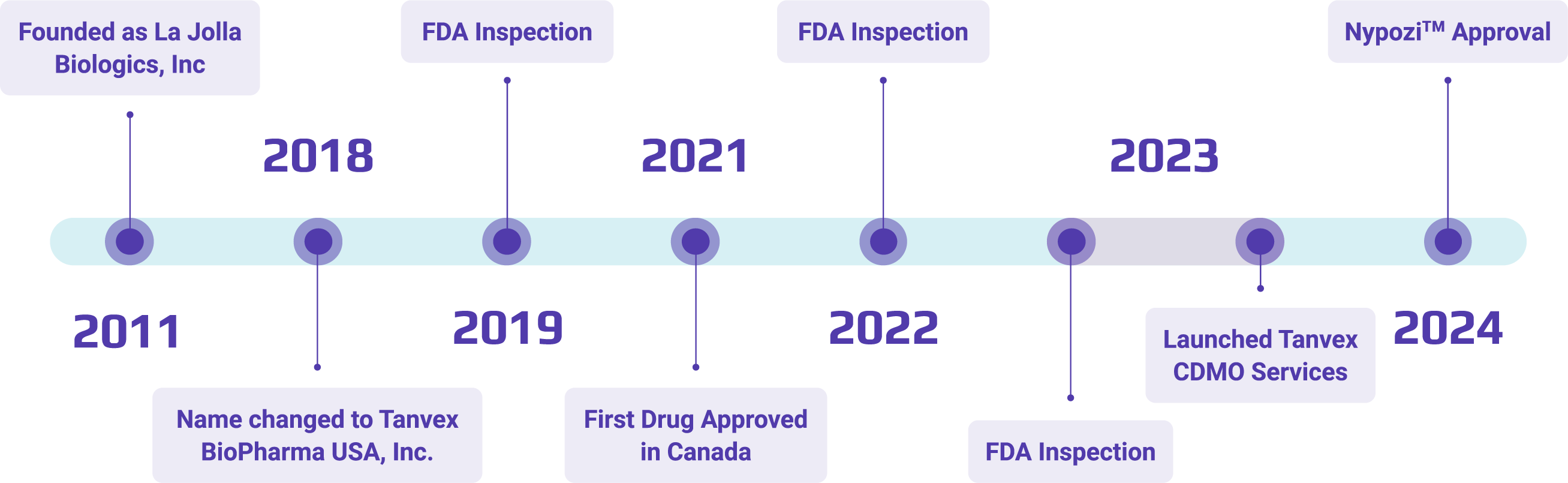
Our team is committed to bringing safer, more accessible biologics to patients in need.
We have successfully brought multiple biologics from IND to BLA.
We harness high-throughput workflows to accelerate the development and optimization of biologic development processes.
Our team operates as a seamless extension of yours throughout every phase of development.
Our team boasts a collective experience of over 100 years in microbial and mammalian biologics development and manufacturing.
Our 100,000 sq. ft. campus houses Research and Development and GMP manufacturing all under one roof for seamless efficiency.

Resources
Explore upcoming events, news, and the latest insights from our team.
Brochure
Read More
›Article
In a new article, Anke Hartung, Ph.D., Associate Director of Bioassay Development at Tanvex CDMO, presents a case study showcasing Tanvex CDMO’s tailored approach to confirming biosimilar functionality. Key highlights: Detailed Glycan Analysis in Biosimilar Development Enhanced Assay Development for Functional Evaluation of Biosimilars Comprehensive Platform Solution for Fc Binding Assays Access the article … Continued
Read More
›Press
Read More
›
Careers
Shape the future of biopharmaceuticals with Tanvex. As we forge ahead in our mission to redefine excellence in biopharma manufacturing, we invite you to explore career opportunities that spark innovation and drive transformative change. Whether your expertise lies in research, development, manufacturing, or beyond, your contributions will resonate on a global scale. Join us in our quest to make accessible, effective, and affordable biopharmaceuticals a reality for all.